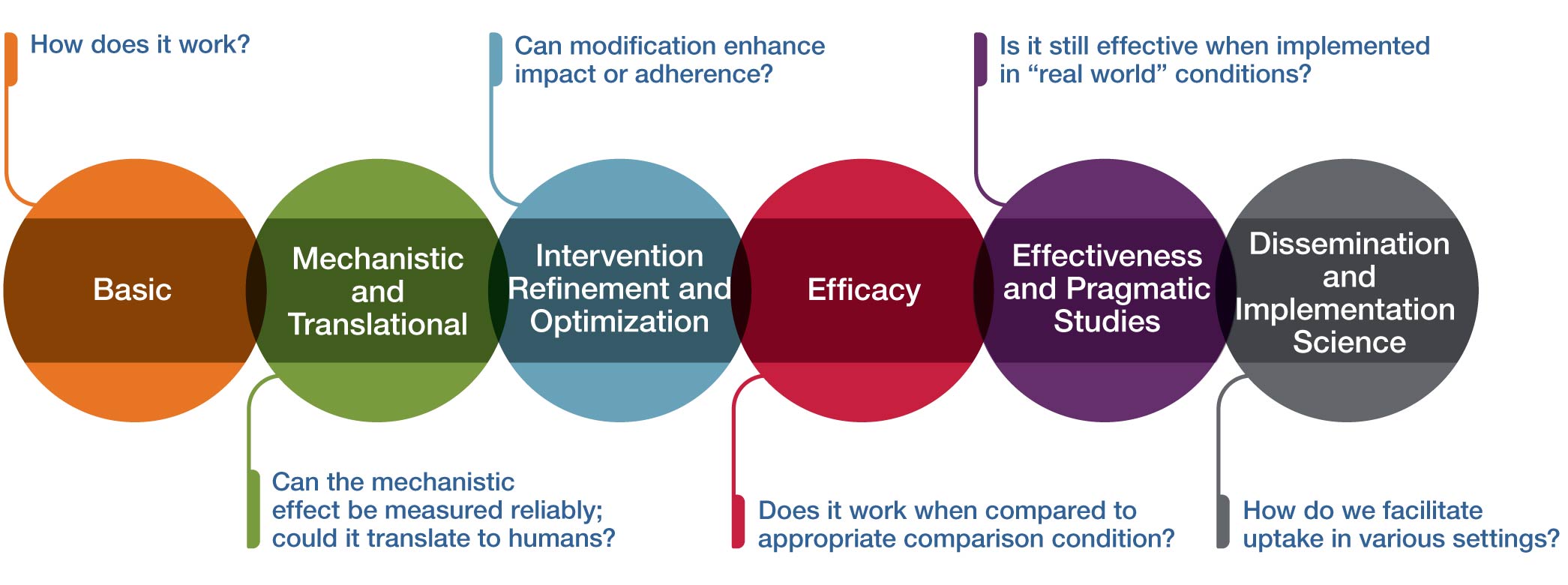
NCCIH Clinical Trials Funding Opportunities
The National Center for Complementary and Integrative Health (NCCIH) no longer accepts most clinical trial applications through the Parent R01 (NOT-AT-20-001). Instead, NCCIH has published a series of clinical trial-specific Notices of Funding Opportunities (NOFOs) for investigators to use instead of the Parent R01. These NOFOs focus on 1) mind and body intervention studies and 2) natural product studies, targeting support of all phases of clinical intervention development.