Objective 1: Advance Fundamental Science and Methods Development

Fundamental scientific inquiry is essential to the progress of biomedical research because it enhances the understanding of how living systems work. This understanding serves as a foundation for translational and clinical studies that can lead to improved approaches for the management, treatment, and prevention of numerous symptoms and conditions and an ultimate restoration of health.
NCCIH’s basic research seeks to understand the nature and scientific principles of complementary health approaches such as their biology, physiology, and physical, chemical, and behavioral properties. This includes research on basic physiological and pathophysiological mechanisms relevant to complementary and integrative health. It also includes identifying and understanding the active components of a complementary health approach and how these components produce effects. Depending on the question, basic and mechanistic studies may be performed in the laboratory, in experimental models, or with human volunteers. The development of tools, models, measures, and methodologies for performing these investigations is at the cornerstone of NCCIH’s mission.
From the outset, complementary and integrative health research has addressed and met methodological challenges stemming from the recognition that natural products are complex mixtures, and that interventions such as yoga involve both contemplative and movement practices. Given the complexity of the approaches we study, the development of sound research design and analytic methods is vital to NCCIH’s mission.
STRATEGIES
Strategy 1: Advance basic and mechanistic research relevant to nutritional, psychological, and/or physical approaches.
Nutritional approaches
NCCIH has a broad interest in studying the biological activities of natural products, such as prebiotics, probiotics, dietary supplements, botanicals, and vitamins. A strong research emphasis is placed on products for which there is compelling preclinical evidence for potential biological activity that may lead to a health benefit or treatment intervention, and/or products that are widely used by the American public. Many of the natural products used by individuals are complex, with multiple molecular constituents that may contribute to their effects. To fully understand the activity of complex mixtures, it is necessary to identify the individual components responsible for a specific activity and determine how those components interact with other components and biological targets. Preclinical model systems are valuable for these studies. Clinical trials of natural products are maximally informative if they incorporate well-formulated biological hypotheses, are built on a sound foundation of basic mechanistic and pharmacologic understanding, and incorporate assessment of defined signatures of biological effects. Thus, the design of maximally informative clinical efficacy trials of natural products requires mechanistic insight as a first step.
Story: The CARBON Program
Plants and plant-derived products are widely consumed for basic nutrition, to promote health and well-being, and for medicinal purposes, worldwide and in the United States. Despite this prevalent use, the mechanisms of action and efficacy of many of these products have not been rigorously evaluated, and the challenges of doing research on these complex materials continue to slow progress toward understanding their contributions to public health. The Consortium for Advancing Research on Botanicals and Other Natural Products (CARBON) Program was launched in 1999 to support research into the safety, effectiveness, and mechanisms of action of botanical dietary supplements that have a high potential to benefit human health.
The CARBON program had its origins with a small number of Botanical Research Centers funded originally in 1999 in response to a Congressional mandate to the Office of Dietary Supplements (ODS) to initiate a program to support botanical research. NCCIH has been a partner on this program from the beginning. Together NCCIH and ODS funded Botanical Research Centers that were tasked with identifying and characterizing botanicals, assessing the chemical components of botanicals, exploring their mechanisms of action, conducting preclinical and clinical evaluations, and training the next generation of scientific researchers. NCCIH and ODS continue to shape the program to tackle the scientific gaps in the field while also addressing shared research priorities. In 2015 and 2020, new components were added to the program focusing on development of novel technology looking at how natural products can affect the many features of cells and specific proteins and on the development of a new nuclear magnetic resonance (NMR) data repository. These and other innovative approaches will break through existing bottlenecks that have hampered progress in natural products research. These additions have ushered in a more collaborative environment for the program where the Centers work closely with each other on specific projects.
In the 20-year history of the program, the Centers have provided rigorous scientific data on the usefulness of a wide range of botanical products, generated research resulting in hundreds of peer-reviewed publications, and trained numerous early-stage scientists. Many of the botanical supplements studied in these Centers–such as black cohosh, bitter melon, chasteberry, fenugreek, grape seed extract, hops, maca, milk thistle, licorice, and valerian–are among the top 100 supplements consumed in the United States based on sales data. The data generated from these and other studies have helped expand our knowledge of natural products.
NCCIH will continue to support research on isolated natural product compounds as well as on the complex mixtures from which they originate. Studies may also focus on both the potential beneficial and harmful effects of natural products, including their interactions with medications. NCCIH-supported studies may also include the characterization of novel natural products or discovering the biological activity of chemical constituents in a complex mixture.
The possibilities of drug interactions, direct toxicities, and contamination with active pharmaceutical agents or environmental chemicals are among the safety concerns about dietary and herbal supplements. Although there is a widespread public perception that herbs and botanical products in dietary supplements are safe, research has demonstrated that these products may carry the same dangers as other pharmacologically active substances. Interactions may occur among prescription drugs, over-the-counter drugs, dietary supplements, and even small molecules in food–making it a daunting challenge to identify all interactions that are of clinical concern. While studies in human subjects are the only way to establish definitive evidence of a clinically relevant drug interaction, the justification for the investment in such a trial is often built on in vitro data.
For example, NCCIH is supporting a Center of Excellence for Natural Product Drug Interaction Research that is focused, in part, on conducting rigorous human subject studies to establish the clinical relevance of interactions for selected natural products. NCCIH also supports rigorous screening of natural product libraries in assays with clear relevance to human metabolism for evidence of pharmacokinetic interactions. The data generated will provide additional information on potential interactions and will help inform prioritization strategies regarding which natural products may warrant future investments in clinical studies.
NCCIH will also continue to support research to elucidate the effects of probiotics and prebiotics on the microbiota naturally present in the human body. NCCIH seeks to address fundamental knowledge gaps, including those pertaining to molecular mechanisms of action of the microbiota and potential interactions with pre- and probiotics and their impact on processes in the human body. NCCIH will continue to work closely with other NIH Institutes, Centers, and Offices; the U.S. Food and Drug Administration (FDA); and the U.S. Department of Agriculture to leverage its investments in this research area.
Psychological and physical approaches
Among complementary physical and psychological approaches are mindfulness-based cognitive therapy, tai chi, yoga, acupuncture, massage, spinal/joint manipulation, art therapy, music therapy, dance, meditation, mindfulness-based stress reduction, and many others. These approaches are widely used by the public and may help meet the need for nondrug approaches for the management of pain and other common, troublesome symptoms, which may benefit from a range of interventions that are safer than drugs and have fewer adverse effects. They may also play a role in interventions to optimize health. Included within the physical and psychological approaches are the qualities of patient–provider relationships and patient engagement. However, there are gaps in the understanding of the mechanisms by which these approaches exert their effects, and this has made it difficult to determine whether they are well suited for specific conditions or target populations and for differentiating responders from nonresponders. The complexity of many physical and psychological approaches has also been a barrier to understanding their effects. NCCIH seeks to support the investigation of the fundamental science relevant to physical and psychological approaches, including mind/brain-focused practices (e.g., meditation, relaxation techniques , hypnosis), body-based approaches (e.g., acupuncture, massage, spinal/joint manipulation/mobilization), meditative exercise (e.g., yoga, tai chi, qi gong), art and music therapies, or integrative approaches combining several components. This may include rigorous fundamental science on less well-studied aspects of the body (e.g., human biofield, hormesis) that may be relevant to complementary therapies.
Mechanistic research on mind and body approaches can address three key aspects. The first is the approach itself: What components impact the biological system or subjective experience? The second is the biological system potentially targeted by the approach: What cellular systems or hormonal, genetic, or neural mediators, for example, are influenced by the intervention? The third is the mechanisms: What are the key processes (i.e., biological, biophysical, and/or behavioral) by which the approach exerts its effects?
Strategy 2: Develop methods, tools, and technologies to study complementary health diagnostic, treatment, and prevention modalities and systems.
NCCIH’s clinical research currently supports trials of both natural products and mind and body interventions and includes early- and mid-phase testing to assess biological signatures of these interventions in humans (and replication of these effects), define appropriate dosage, refine the components and system of intervention delivery, determine optimal frequency or duration of the intervention, assess feasibility, and enhance adherence. The Center also supports later stage full-scale efficacy, effectiveness, or pragmatic trials when the evidence base is sufficient to justify them.
Rigorous research on complementary health approaches requires well-established methodologies, including valid, reliable, and relevant research tools and outcome measures. NCCIH seeks to support the development of improved quantitative and qualitative methodologies for complementary health research, especially those that can be used to assess symptoms, multisystem interactions, patient engagement, health restoration, and resilience. Studies that identify and validate objective endpoints or biomarkers predicting therapeutic response, assess and measure adherence or treatment fidelity, or otherwise strengthen the design of clinical trials of complementary health approaches are particularly important.
NCCIH is also interested in the study of multicomponent systems including those with diagnostic and therapeutic frameworks different from those of conventional medicine. Studying these systems is more difficult than studying individual treatment modalities. However, if done with appropriate scientific rigor, it could help inform or complement areas of conventional medicine, as well as the systems of care themselves. Research on this topic might begin with studies to test the reliability and validity of complementary diagnostic systems. The development of rigorous and reproducible treatment protocols is also needed for use in clinical trials to assess their efficacy.
Catalyze advances in natural products methodology
Natural products have a long and impressive history as sources of medicine and as important resources for biological research. However, many of the techniques for studying complex mixtures of natural products have remained unchanged for many years and have yet to leverage advances in biological and chemical methodologies.
To move the field forward, NCCIH is emphasizing research to overcome methodological and technological hurdles that hinder advances in natural products research. For example, omics-based and other high-throughput technologies may help researchers evaluate the validity of hypothesized additive or synergistic effects that are at the core of many traditional herbal medicines. In addition, the use of network pharmacology–the study of the web of biologic targets for any bioactive substance–will enable researchers to investigate the complex effects of natural products on multiple targets in ways that were not possible before.
NCCIH is supporting the Natural Products Magnetic Resonance Database (NP-MRD), an electronically accessible data repository allowing key information about the world’s natural products to be openly shared and rapidly queried by the global scientific community. It will be particularly important for those scientists using NMR spectroscopy to study materials of natural origin to identify new products that may one day be used to improve health or cure disease. The NP-MRD will become an important hub for natural product chemists around the world, allowing them to share their data, learn from each other, and accelerate the translation of their discoveries to improve health.
Support development of technologies and instruments for clinical research on physical and psychological approaches
The goal of many studies of mind and body interventions is to optimize their practice and delivery to maximize efficacy. This may be accomplished through technological innovation to monitor and possibly facilitate relevant underlying processes associated with these interventions. For example, NCCIH is interested in the development and/or pilot testing of devices to provide biofeedback or optimize practice, wireless technologies for real-time data collection and monitoring of brain activity or other physiological signals, biochemical or epigenetic monitoring devices, and electrodermal monitors. It is also important to optimize and pilot test components of physical and psychological approaches for their mechanistic effects on biological processes. The development of a patient-report measure to assess aspects of the healing context such as beliefs, expectations, perceptions of the patient–provider relationship, and other aspects of the overall healing environment will advance research on complementary and integrative health approaches, will shed light on understudied phenomena such as placebo responses, and may ultimately contribute to improvement in research trial design. With the increase in telehealth over the past year, NCCIH is also interested in the development and optimization of technologies for home-based and remote delivery of physical and/or psychological approaches.
Test the reliability and validity of complementary diagnostic systems
Complementary diagnostic systems may be different than those of conventional medicine and focus more on the prevention of disease and restoration of health. While it is important to study specific therapies, it is also important to develop techniques to study a system of care, such as traditional Chinese medicine, chiropractic, Ayurveda, homeopathy, or naturopathy, to determine the reliability of its diagnostic methods. Research design must account for complexity if the diagnostic system will attempt to personalize results or recommend interventions based on individual characteristics and be validated with rigorous, reproducible studies. Validation of these diagnostic systems may involve the use of omics-based technologies to define the cellular, molecular, and immune changes in response to treatments. Retrospective studies may be important to determine the extent to which key principles of complementary diagnostic systems are implemented in practice.
Complementary Therapies
Nutritional, physical, or psychological approaches
May have originated outside of conventional medicine
Multicomponent Interventions and Systems
Multicomponent interventions combine multiple approaches that address different aspects of a person
Multicomponent systems may use diagnostic and therapeutic frameworks that are different from those of conventional medicine
Define treatment algorithms for complementary interventions and systems and establish their fidelity and reproducibility
Studying complementary systems is going to require the development of reproducible intervention models, new methodologies, and outcome assessment measures for study in rigorous clinical trials. This process can also be termed “manualization,” where a treatment manual is developed through collaboration between practitioners and researchers. The manual provides guidelines for diagnosis and treatment using complementary systems. This approach allows for standardization of the system and facilitates analysis and reproducibility. Different approaches to analysis of outcomes may need to be employed in research on complementary systems. For example, the real-world perception of an individual regarding the benefits of treatment may be important in this context. NCCIH is interested in pragmatic efficacy or effectiveness trials to test the effects of a manualized intervention.
Develop, refine, and test clinical research models and relevant statistical methods for testing multicomponent interventions and systems
There is a need for research to evaluate multicomponent interventions as they are used and delivered to determine whether they are safe and effective. For clinical trials to address this need, they must be well designed and test hypotheses that will guide decisions about the inclusion of a multicomponent approach in the delivery of health care for a specific condition. To that end, it is typically necessary to conduct a series of early-phase clinical trials to gather the multiple types of preliminary data needed to design subsequent large and rigorous efficacy or effectiveness studies. Although the scientific literature may provide the rationale for conducting an efficacy or effectiveness trial, investigators often lack critical information about key variables needed to implement a complex intervention in a clinical trial. Some key aspects that may need further investigation to plan the future trial could include finalizing the multicomponent intervention or system delivery method, the outcome measure(s), or the recruitment strategy necessary to design an efficacy or effectiveness trial. Early-phase clinical trials can fill this information gap, thereby improving study design and knowledge of whether a complex intervention can be implemented in a trial with fidelity and reproducibility; whether participants will adhere to the multicomponent intervention; and the overall feasibility of the trial. Later phase trials can further explore, develop, and test adaptive interventions; optimize or tailor a multicomponent intervention to have a greater impact on the potential mechanism of action; assess whether the multicomponent intervention can be delivered with fidelity across sites; identify effective ways to recruit sufficient participants from relevant populations across sites; or determine the optimal duration or frequency of the intervention to be used in the future multisite trial. This approach may require several stages of research and development from basic science to intervention generation/refinement/modification/adaptation and pilot testing to efficacy, effectiveness, dissemination, and implementation research. In addition, multicomponent interventions and systems may require innovative trial designs and advanced statistical methods to explore which components are necessary and/or sufficient for a clinical effect and to look at the impact of the multicomponent intervention on multiple systems or composite outcome indices.
There are statistical challenges in studying integrated multicomponent therapies and systems. Composite scales, such as the Charlson comorbidity index or measures of health-related quality of life, can be helpful in assessing treatments in longitudinal studies. Composite scales do not necessarily require larger sample sizes. Factor analysis and principal component analysis can also be used in scale development. Real-world observational “big” data can be used for scale development, guideline development, clinical trial design, and hypothesis generation but are not suited for evaluating causal relationships. Machine learning using big data has potential for classifying and clustering patients, including identifying subpopulations of complex patients who may benefit from targeted care management strategies. The development of systems science and integrative physiology methods will also be important to further understanding the impact of multicomponent therapies on multiple systems.
NCCIH Framework for Clinical Research
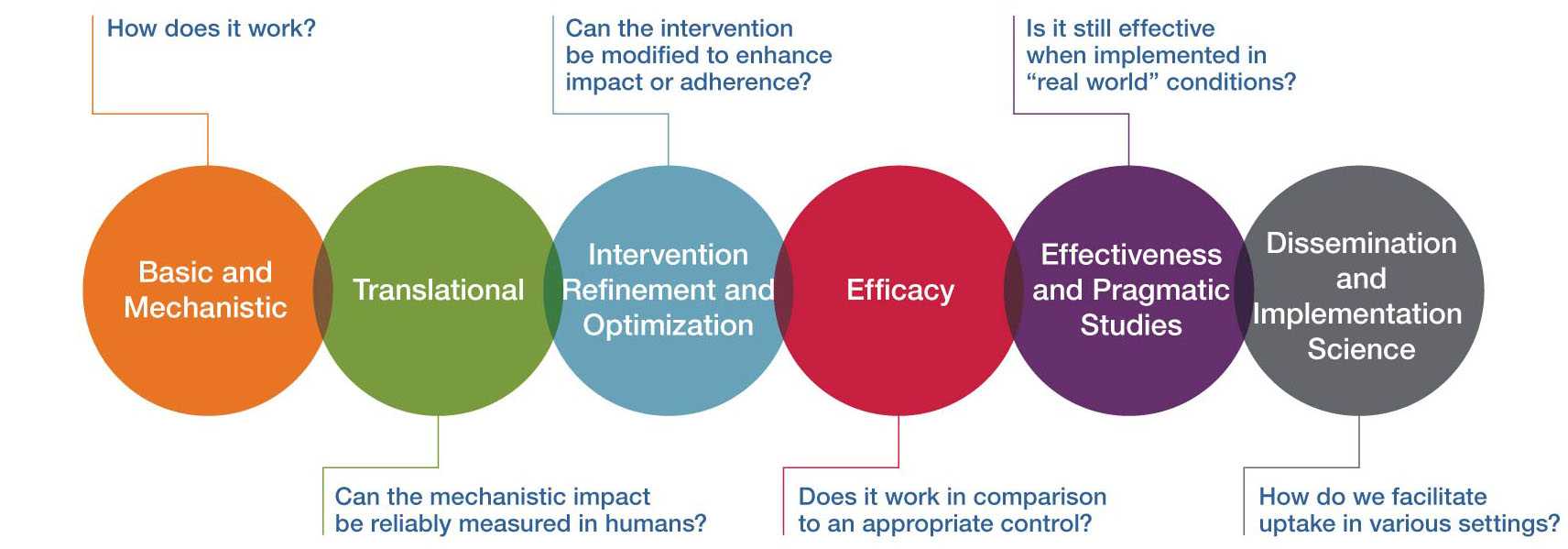
Strategy 3: Develop outcome measures to quantify health restoration and resilience.
It is difficult to quantify health, health restoration, and resilience. Validated outcome measures are needed if research is to advance in this area. Many scales to measure resilience have been published, and it is important to further examine these and to develop new outcome measures to quantify both physical and psychological resilience. The development of outcome measures for health restoration must consider what is important to each person in terms of restoring their own health. Also important is the development of technology and outcome measures for mechanistic studies (e.g., data from wearables could be used to determine the relationship to subjective self-report measures).
Strategy 4: Develop methods to conduct implementation science and effectiveness research on complementary and integrative health approaches.
Published results of efficacy and effectiveness studies on complementary health approaches should lead to widespread uptake of evidence-based practices, but too often, the scientific pathway ends prematurely, before the best ways to improve adoption, implementation, and sustainability can be determined. NCCIH supports the full continuum of the research pipeline, whereby a complementary health intervention moves from basic and mechanistic research, through efficacy trials, and through dissemination and implementation, including systems-level research addressing access and reimbursement for interventions. Whereas efficacy and effectiveness studies are designed to answer the question, “Which intervention(s) should we use?” dissemination research asks, “Are the relevant clinicians and target population aware of the novel evidence-based intervention(s)?” Implementation science focuses on “How can these novel evidence-based intervention(s) be more widely and rapidly used in practice?” It should be noted that for complementary and integrative health, the novel evidence-based intervention may be an existing intervention used in a novel setting (e.g., use of acupuncture in a hospital emergency department). The goal is to decrease the time between establishing the evidence base of interventions and the widespread uptake and adoption of these interventions. The development of methods to conduct implementation science and effectiveness research on complementary and integrative health approaches is a high priority for NCCIH.