HEAL Initiative Offers New Funding Options for Clinical Trial Research on Pain
December 21, 2018
Are you interested in taking advantage of the NIH HEAL InitiativeSM funding opportunity announcements (FOAs) to conduct a clinical trial that evaluates pain treatment without a specific focus on opioid use disorder? The National Center for Complementary and Integrative Health (NCCIH) has several for you to consider depending on stage of intervention development and testing. This blog describes how to choose from available mechanisms for initial efficacy testing, effectiveness research, and pragmatic implementation science for pain treatment.
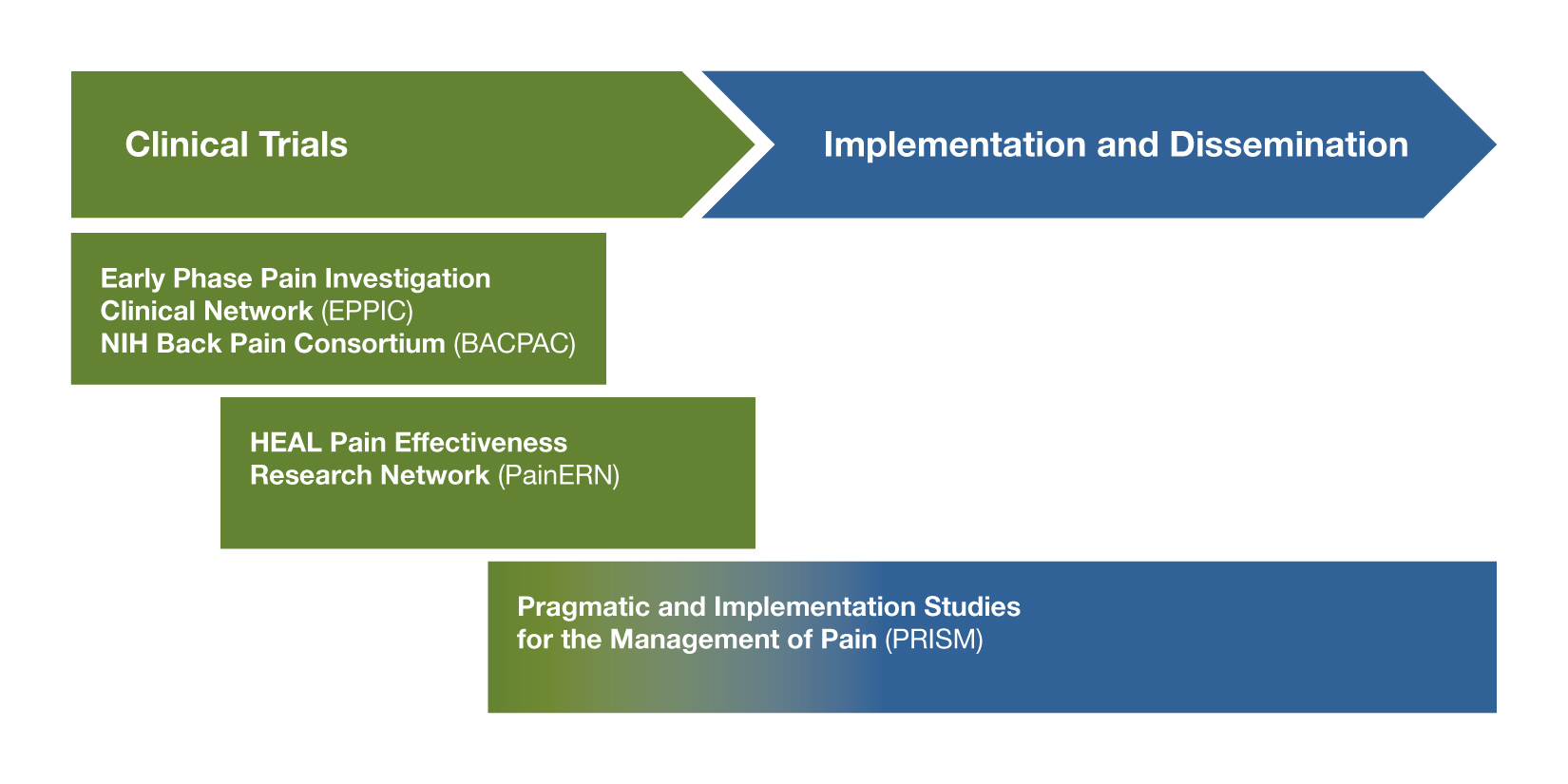
Investigators Interested in Early Stage Clinical Research: Does the Novel Intervention Have Promise for Future Trials?
The Early Phase Pain Investigation Clinical Network (EPPIC; RFA-NS-19-025)
If you are interested in testing safety and developing proof of concept data for novel nonaddictive therapies for pain, consider applying to participate in the EPPIC-network. The EPPIC-network will provide infrastructure for in-depth phenotyping and biomarker studies in patients with specific pain conditions, as well as rapid design and performance of Phase 2 clinical trials to test the safety and collect early clinical data for promising novel therapeutics for pain (devices, biologics, and drugs). NCCIH encourages applications for investigators to serve as “hubs” for the EPPIC-Net clinical network. The treatment of low-back pain is a specific area of focus.
Scientific contact: Dr. Inna Belfer
NIH Back Pain Consortium Research Network (BACPAC; RFA-AR-19-029)
The BACPAC research program will fund Phase 2 trials on interventions for chronic low-back pain, including complementary therapies, conducted within the infrastructure of the EPPIC-network. NCCIH encourages investigators wishing to test the effects of novel complementary and integrative approaches or multimodal approaches that include complementary approaches for low back pain to apply to the BACPAC FOA. For more information, consider attending a BACPAC pre-application webinar.
Scientific contacts: Dr. Wen Chen and Dr. Wendy Weber
Investigators Interested in Effectiveness Research: Does the Intervention Work in “Real World” Conditions? Which Intervention Works Better for a Particular Pain Condition?
The Pain Management Effectiveness Research Network (HEAL PainERN; RFA-NS-19-021)
For interventions already supported by published safety and efficacy data, the HEAL PainERN will support comparative effectiveness trials of existing therapies or effectiveness of existing or novel approaches for preventing and managing pain while reducing risk of addiction. The overall goal is to inform clinicians about the effectiveness of interventions or management strategies to improve outcomes across the continuum of acute to chronic pain. Awardees will use the infrastructure of the National Center for Advancing Translation Sciences (NCATS) Trial Innovation Network, which will provide clinical coordination center services, data coordination center services, recruitment and retention services, and biostatistical support. The HEAL PainERN is described in more detail on the NIH Pain Consortium website. This funding opportunity is not appropriate for initial efficacy testing. NCCIH is interested in comparative effectiveness trials of mind and body approaches that have already demonstrated efficacy for specific pain conditions.
Scientific contact: Dr. Lanay Mudd
Investigators Interested in Implementation Research: When the Intervention is Implemented in Health Care Settings, Does It Still Work?
Pragmatic and Implementation Studies for the Management of Pain to Reduce Opioid Prescribing (PRISM; RFA-AT-19-004)
Investigators are encouraged to apply to the PRISM program to study the integration of interventions with demonstrated efficacy for pain treatment into health care delivery with partnering health care systems, or to study the implementation of health care system changes to improve adherence to evidence-based pain management guidelines. The PRISM FOA requires that the intervention be embedded into three or more health care systems, and should use pragmatic methods (e.g., clinic level randomization, primary use of electronic health care record data, no or unobtrusive adherence monitoring, etc.). Awardees will become part of the NIH Health Care System (HCS) Research Collaboratory and will work with the established HCS Clinical Coordinating Center to facilitate the planning and conduct of the study. NCCIH encourages applications that will study the integration of complementary health approaches with demonstrated efficacy or effectiveness for pain treatment into health care delivery, as well as applications that will study methods to improve adherence to evidence-based pain management guidelines that include complementary and integrative health approaches. For more information, consider attending a PRISM pre-application webinar.
Scientific contact: Dr. Wendy Weber
Pragmatic Randomized Controlled Trial of Acupuncture for Management of Chronic Low Back Pain in Older Adults (Acupuncture; RFA-AT-19-005)
Applications are encouraged to conduct an efficient, large-scale pragmatic trial to evaluate the impact of, and strategies to best implement, acupuncture treatment of older adults (65 years and older) with chronic low-back pain. This FOA requires that the intervention under study be embedded into health care delivery systems. Trials must be conducted across two or more health care systems and will become part of the NIH HCS Research Collaboratory. For more information, consider attending an Acupuncture pre-application webinar.
Summary
We hope this blog clarifies the differences between these exciting HEAL Initiative opportunities for clinical trial research on pain treatment. Investigators should read the FOAs for more information. Application deadlines are as follows:
- HEAL PainERN (RFA-NS-19-021): February 1, 2019
- EPPIC (RFA-NS-19-025): February 6, 2019
- PRISM (RFA-AT-19-004): February 8, 2019
- BACPAC (RFA-AR-19-029): February 26, 2019
- Acupuncture (RFA-AT-19-005): March 15, 2019
View NCCIH’s Role in the NIH HEAL Initiative to see a full list of FOAs that we are supporting.


Comments
Comments are now closed for this post.