The R34: An Opportunity for Critical Groundwork in Clinical Trials Research
March 24, 2015
Which funding mechanism should be used for early stages of clinical research? As Chief of the Clinical Research in Complementary and Integrative Health Branch and formerly a Program Director, I've spoken to many investigators about choosing an appropriate funding mechanism for a proposed clinical study. I've also seen applications for mind and body clinical research that, unfortunately, lack critical preliminary data. Often, I'll encourage investigators to make a list of the preliminary data that would help them design a future efficacy or effectiveness trial. Once they have this list, they can prioritize and identify the most important issues that need to be addressed. Sometimes, the R34 is just the right grant program to assist researchers gather the data they need.
I would like to share NCCIH’s Framework for Developing and Testing Mind and Body Interventions—and show you how it relates to the R34 planning grant.
The framework consists of six major stages that apply to mind and body clinical research and are graphically represented by six colored circles:
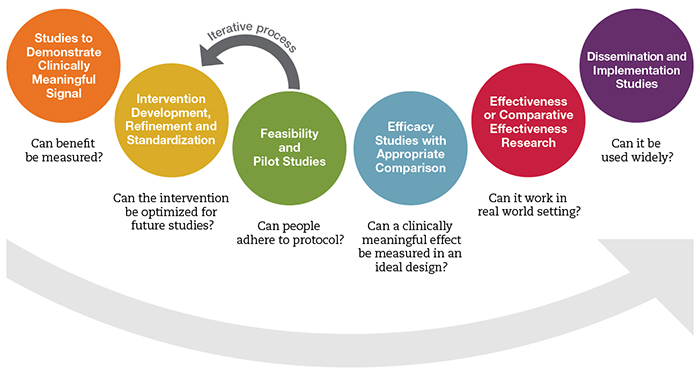
In the orange circle (i.e., the first stage), the question being addressed is whether the intervention of interest can demonstrate a biologically and/or clinically meaningful and measurable effect when employed. Often, our Program Directors hear from investigators who have conducted a small pilot study at this stage (such as a case series, controlled or uncontrolled, with 10 to 20 participants). The researchers have performed a pre- and post-analysis, and have found a clinically meaningful signal.
Investigators sometimes want to make the leap to apply for a study in the blue or red circles (i.e., an efficacy study with appropriate comparison, or an effectiveness/comparative-effectiveness study) without first having conducted research in the yellow and green circles (respectively: intervention development, refinement, and standardization; and feasibility and pilot studies). This leap to the efficacy/effectiveness stage can have unintended consequences of conducting an inadequately powered study with an intervention that has not been refined for study and in a population that may not include those most responsive to the intervention.
The framework has an important relationship to NCCIH’s current R34 opportunity, Exploratory Clinical Trials of Mind and Body Interventions for NCCAM High Priority Research Topics, as follows:
- The R34 is designed to support work in the yellow and green circles.
- It is ideally positioned for researchers who have done a small pilot study that demonstrates a signal that an intervention may have benefit for a particular condition. Yet, additional data is needed to justify the design choices for the future clinical trial.
- If this is your situation, it is good to think about how you would design an eventual, larger-scale effectiveness or efficacy study. In order to maximize your chances of being able to recruit and complete that future definitive study, consider conducting an additional study using the R34 mechanism.
What are some advantages of using the R34 funding opportunity?
- You can obtain data critical for planning and defining a subsequent, full-scale clinical trial.
- You can obtain data useful in filling gaps in scientific knowledge and necessary for a trial to be competitive.
- You can further refine and develop the intervention to be sure you are delivering it in a way that will have high adherence and will be of sufficient duration to have an impact. This may require assessing how often and how long the intervention should be delivered. If appropriate, you can also assure that the intervention can be delivered with fidelity by a variety of well-trained instructors.
- You can develop and test which comparison conditions are most acceptable and have similar retention and adherence as the test intervention.
- If the future clinical trial would require a large sample size, you may need multiple sites. You could propose an R34 to pilot-test the intervention at multiple sites, demonstrating recruitment, data collection, and fidelity across the sites.
- You will be able to demonstrate that the intervention is being studied in the right population, and is being delivered in the way most likely to obtain the best adherence, retention, and participation. You will also be able to demonstrate your team’s ability to recruit and retain participants representing those who have the condition under study.
I encourage you to consider the various stages of clinical trials research and use of the R34 mechanism. NCCIH Program Directors are available to discuss your research interests, questions, and approaches. They can also advise you on available or appropriate funding mechanisms for your research.

Comments
Comments are now closed for this post.