New Study Identifies Mechanism Underlying Wound Healing and Potential Target for Speeding Healing Process
The ion channel PIEZO1, which spans cell membranes and helps convert mechanical forces into electrochemical signals, regulates skin cells called keratinocytes during wound healing and may be a target for developing medicines that speed up the healing process, according to a new study published in the journal eLife. The study, partially funded by the NIH New Innovator Award and supported by the National Center for Complementary and Integrative Health (NCCIH), was conducted by researchers at Scripps Research and the University of California, Irvine.
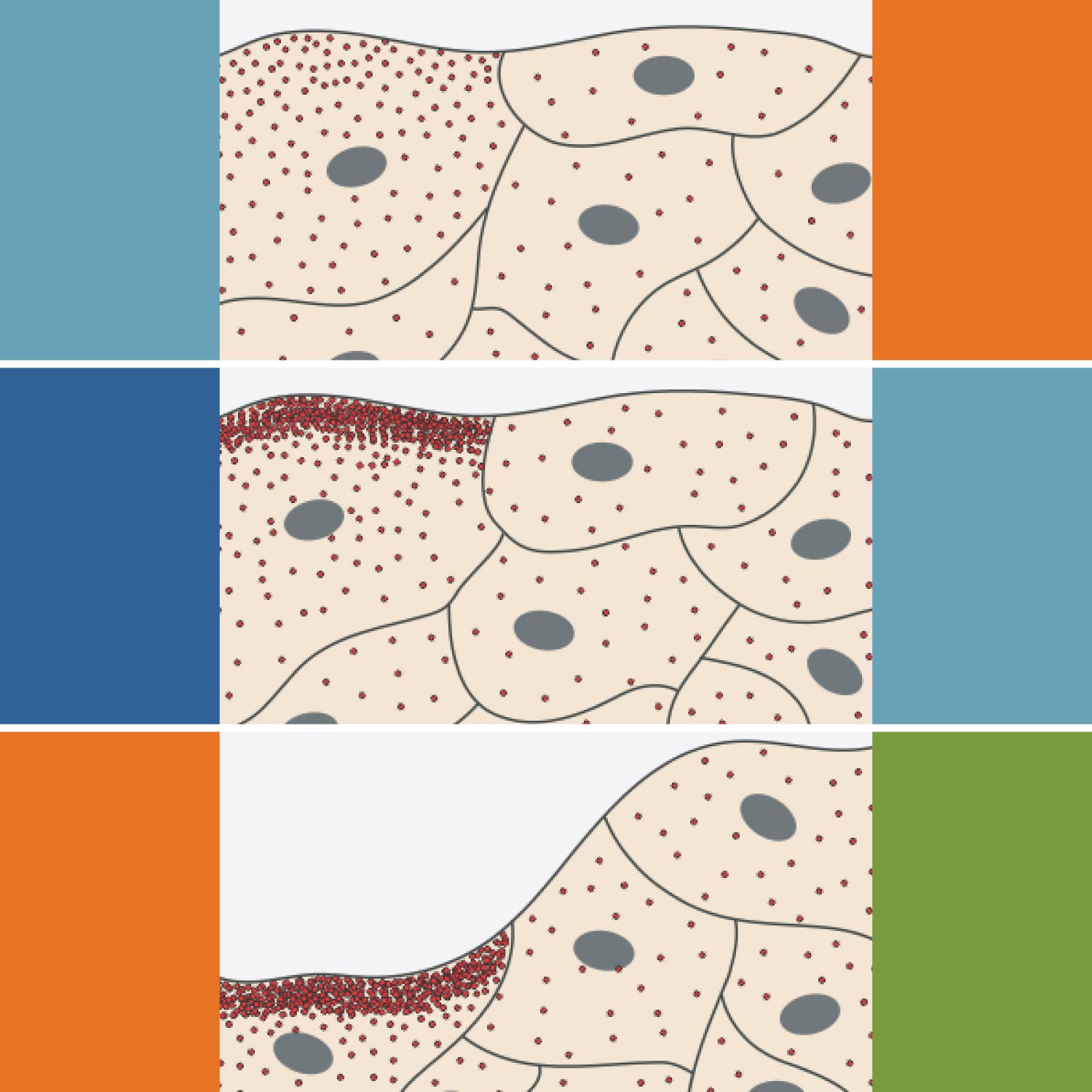
During the repair of wounded skin, keratinocytes—the predominant cell type in the outermost layer of the skin—migrate from the wound edge into the wound, where they play an essential role in regenerating the skin. Mechanical forces are known to regulate keratinocytes as they restore the skin. Now, new research has identified the biophysical mechanism underlying this process, showing that the mechanically activated ion channel PIEZO1 plays a key role.
Through molecular, cellular, and organismal studies, researchers showed that PIEZO1 activity slowed wound healing. Mice with a mutation increasing the flow of ions through PIEZO1 channels had slower wound closure than control mice. Genetically altered mice with damaged PIEZO1 channels in the skin had faster wound closure than control mice.
Imaging of PIEZO1 channels over time showed increased activity of channels at some wound edge regions, leading to a localized retraction of keratinocytes that slowed the cells’ collective migration into the wound bed. Within individual keratinocytes, more PIEZO1 channels were active at the rear of the cell, where maximal retraction occurs. Chemical activation of PIEZO1 channels enhanced both single and collective keratinocyte retraction, further supporting that PIEZO1 activity slows keratinocyte migration and the healing process.
These findings suggest that a topical medicine inhibiting PIEZO1 ion channels could help speed wound healing, potentially reducing the risk of infection. The authors note, however, that the quality of wound healing after PIEZO1 inhibition still needs to be assessed; slower healing may have advantages. The authors say it is also important to determine whether inhibiting PIEZO1 channels has any negative effect on normal sensation of mechanical stimulation or on other important functions in keratinocytes.
On a broader perspective, the authors noted, the study’s findings have implications beyond wound healing to processes like development, homeostasis, disease, and repair.
Reference
- Holt JR, Zeng W-H, Evans EL, et al. Spatiotemporal dynamics of PIEZO1 localization controls keratinocyte migration during wound healing. eLife. 2021;10:e65415.
Additional Resources
Publication Date: November 10, 2021